Education for runners and endurance athletes. Learn to train smart, run fast, and be strong.
These articles are free.
Please help keep them alive by buying me a beer:
 Buy me a beer.
Buy me a beer.
This article is part of a series:
Why has the simple act of drinking water become the most confusing topic in sports nutrition?
→ Part 1 — What we know
→ Part 2 — What we aren’t sure about
→ Part 3 — What we don’t know
→ Part 4 — What you can do
Why has the simple act of drinking water become the most confusing topic in sports nutrition?
→ Part 1 — What we know
→ Part 2 — What we aren’t sure about
→ Part 3 — What we don’t know
→ Part 4 — What you can do
“Drowning” in hydration. Part 3 of 4:
Hydration during exercise. What we don’t know — The salty dilemma with sodium.
Thomas Solomon PhD.
29th August 2021.
Electrolytes! Sodium! Stay hydrated! Great Scott, how many more companies will stuff “hydration” potions down our face to keep us more “hydrated” for longer? Today is all about sodium and the salty dilemma of whether you need it or not during exercise.
Reading time ~20-mins (4000-words).
Or listen to the Podcast version.
Or listen to the Podcast version.
As you learned in the first part of this series, both water and solutes (like sodium) in your tank continuously move between the intracellular and extracellular pools — between the cells in your organs and your blood — to help maintain plasma osmolality (a kind of “liquid pressure”) and plasma volume. You are now very well versed in the fact that water leaves your “tank” (dehydration occurs) during exercise… but what about solutes? In particular, what about sodium?
It might not surprise you that lots of athletes use sodium during races. For example, a study of 3317 ultra runners competing in 3 races ranging from 65 to 165 km in hot and humid conditions showed that 59% (1957/3317) of the runners had enriched their food or drink with sodium during their race. Meanwhile, a diet record in elite ultra-endurance runners during the 24-h World Champs found a sodium intake of 869±431 mg/hour of sodium (range 271 to 1542). But, before assuming that you should ingest sodium during exercise, you must first ask yourself an obvious question...
A change in plasma osmolality is a good indication that plasma sodium concentration has changed because sodium is the most abundant solute in plasma. So, the first important question to ask is...
 Do we lose sodium in our sweat?
Do we lose sodium in our sweat?
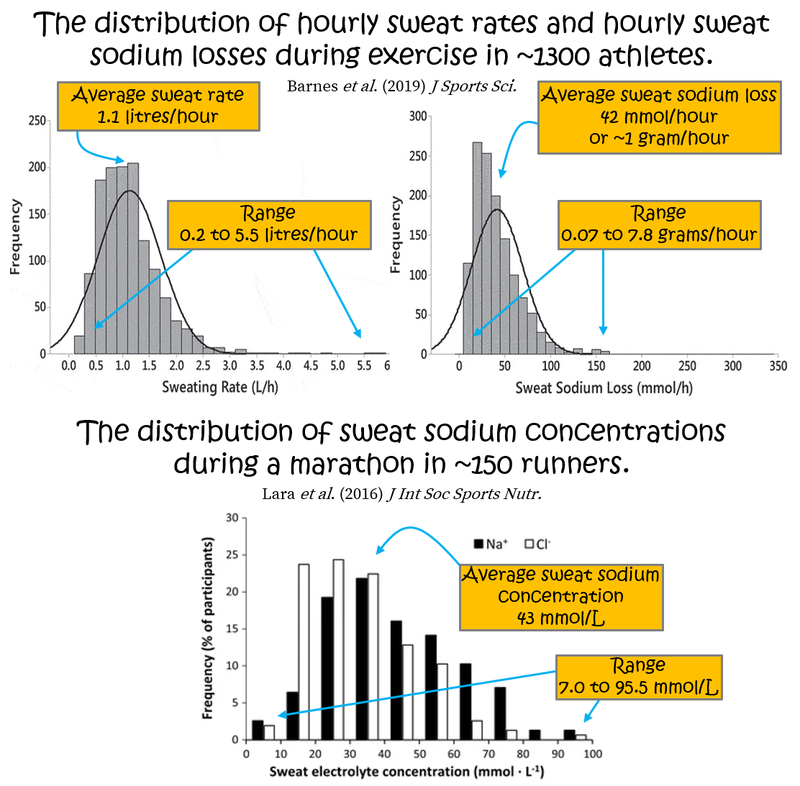
As you know, during exercise, you sweat. Typically, a higher sweat rate is associated with higher sweat sodium concentration because there is less time for sodium reuptake as sweat flows through your sweat glands (see here and here). For a phenomenal deep-dive on sweat glands, their function, and the role of sweat in human health, I can thoroughly recommend Lindsay Baker’s 2019 narrative review on the physiology of sweat gland function and her 2017 review of sweat/sodium loss methodology. Such details are unnecessary here but Lindsay also published a cool epidemiological data set of normative values for sweat rate, sweat sodium, and sodium losses during exercise in ~1300 athletes. On average, sweat rate was 1.1±0.6 litres/hour (mean ± standard deviation), sweat sodium concentration was 36±11 mmol/L (millimoles per Litre), and sweat sodium loss was 42±28 mmol/hour (equivalent to 1.0±0.6 grams/hour). But, these variables show dramatic variability within- and between-people, across many sports including marathon running, with sweat rates ranging from ~0.2 to ~5.5 L/hour, sweat sodium concentrations ~10 to ~90 mmol/L, and sodium losses ranging 3 to 340 mmol/h (equivalent to 0.07 to 7.8 grams/h)!
 Do our plasma sodium concentrations drop during long-duration exercise?
Do our plasma sodium concentrations drop during long-duration exercise?
This is important because if plasma sodium concentrations fall then plasma osmolality falls, which can disrupt normal organ function. Worse, if plasma sodium falls below 135 mmol/L, this indicates hyponatremia and your risk of needing medical care and developing a serious health event requiring hospital admission increases. So, what happens during exercise…
Observational data from ~800 triathletes completing an Ironman in ~17-24°C showed a huge range in body weight loss (a proxy for dehydration) during the race but, on average, plasma sodium concentrations and osmolality increased not decreased. Similarly, observations of 33 athletes racing the 56 km Two Oceans Marathon in South Africa in 2009 showed that total body water decreased (dehydration) while, again, plasma sodium concentrations and plasma osmolality increased but to a small extent and within the normal range.
More intense events, where sweat rates are higher, may affect plasma sodium. For example, data from 51 marathoners racing the 2014 Rock n’ Roll Madrid Marathon in 18 to 29°C found that runners in the highest tertile of sweat sodium concentrations (aka “salty sweaters”) had the lowest post-race plasma sodium concentration and plasma osmolality compared to pre-race values. But, no participant was found to have hyponatremia at the end of the race and the during-race decrease was very small and their plasma sodium (141±1 mmol/L) and osmolality (297±6 mOsm/kg) were still well within the healthy reference ranges. That said, there is massive variability in sweat rates and sweat sodium losses among marathoners so this does not exclude the possibility that hyponatremia can arise during such events in some people.
At the more extreme Western States 100-miler, an event that takes up to 30-hours includes more than 5000 metres of elevation gain, and often hits temperatures above 30°C during parts of the race, a 5-year observation of 887 competitors found just over 10% incidence of hyponatremia at the end of the race (101 of 887 runners). Likewise, observations at the hot (39°C) 2013 Western States race found that 10% of participants gained body mass and 7% were hyponatremic at the finish. Similar examples of hyperhydration have been found during other ultramarathon and multistage races (here and here). Interestingly, however, cases of hyponatremia were not solely found in folks who hyperhydrated and gained weight during the race, indicating that body sodium loss (independent of plasma sodium dilution) may also cause hyponatremia under such arduous conditions.
Don’t worry if concentrations (mmol/L) and absolute amounts (grams) and sweat rates (L/hour) and blah blah blah are all a bit confusing. If it is, think of it like this:
So,
 Yes, sweat contains sodium and you do lose sodium during exercise but this doesn’t necessarily mean that your plasma sodium concentration will decrease because your body strives to maintain plasma sodium (and osmolality) within the normal range during exercise… and it does a great job!
Yes, sweat contains sodium and you do lose sodium during exercise but this doesn’t necessarily mean that your plasma sodium concentration will decrease because your body strives to maintain plasma sodium (and osmolality) within the normal range during exercise… and it does a great job!
 That said, hyponatremia, which is dangerous, can arise from either drinking too much water (hyperhydration) or from excessive sweat sodium losses during exercise, or a combination of both.
That said, hyponatremia, which is dangerous, can arise from either drinking too much water (hyperhydration) or from excessive sweat sodium losses during exercise, or a combination of both.
 And, the chance that your plasma sodium concentration will drop becomes higher with increasing distance (e.g. ultras) especially under extreme conditions (e.g. high ambient temperature).
And, the chance that your plasma sodium concentration will drop becomes higher with increasing distance (e.g. ultras) especially under extreme conditions (e.g. high ambient temperature).
So, if hyponatremia (plasma sodium ≤135 mmol/L) is a hazard with dire consequences, perhaps you are wondering...
To start with something simple, specifically adding sodium increases the “saltiness” of water, which is important because, just like thirst (i.e. water appetite), salt appetite is tightly regulated by neuroendocrine mechanisms in your body (you can go deep on that here and here). So, if you crave something salty during a session/race, drink or eat something salty — doing so will likely quench your thirst better than if you drank water alone (thank you, Coach Klein).
Adding solute also gives water another extra property; adding solute increases the osmolality — the “liquid pressure” — of the water. Why does this matter? Well, water movement through your stomach and intestine is passive and depends on osmotic gradients — water will always move “downstream” from where there is too much to where there is too little. Since osmotic gradients are determined by osmolality, water movement from your drink bottle to your blood is, therefore, influenced by solute concentrations. Drinking a solution with a low sodium and/or glucose concentration (and, therefore, low osmolality) will allow water to “run” into your blood faster. This is why sports drinks are either “hypotonic” or “isotonic” — the osmolality of sports drinks is lower than or equal to the osmolality of your blood. But...
 What does adding sodium (the most abundant solute in your blood) to your water do during exercise?
What does adding sodium (the most abundant solute in your blood) to your water do during exercise?
Some laboratory studies show that sodium intake during exercise may help your body retain fluid by increasing water reabsorption in your kidneys so you pee less. But, this doesn't mean you need to start drinking salty water every time you throw liquid into your pie hole. Plus, the artificial conditions of lab studies are never an ecological model for what really goes down on race day. So, what can we learn from the few “in-the-field” studies…
Observations of 161 athletes competing in an 80 km section of a 6-day 250 km ultramarathon found that all athletes used sodium during the race, ranging from ~2 to ~7 g, but sodium intake was not correlated with running pace, race time, or finish position and, at the end of the race, hyponatremia (plasma sodium ≤135 mmol/L) was less likely but not absent in higher sodium users.
Several observational studies have also been completed at the Western States 100-miler. A survey of 155 athletes who completed the race on a day with a max high of ~32°C showed that sodium supplements were used by ~94% of the runners and that a higher post-race plasma sodium concentration was correlated with a higher rate of sodium intake during the race. The survey showed that sodium intake during the race was not different between folks who had hyponatremia (plasma sodium ≤135 mM) at the end of the race and folks with healthy plasma sodium concentrations at the end of the race, but that hyponatremia tended only to occur in runners with a low sodium intake (less than ~120 mg/hour) during the race. Furthermore, hyponatremia was detected in groups of runners who had either lost or gained weight. Meanwhile, on a hotter day at Western States when the air temperature reached 39°C, runners who supplemented more sodium during the race lost less weight (possibly indicative of less dehydration) but average finish times were not different between participants who used sodium during the race vs. those who did not. Interestingly, runners who hyperhydrated and gained weight during the race had used more sodium (182 mg per hour) than those who lost weight during the race (106 mg/h). Full dietary records from 20 racers that year also showed that sodium intake ranged ~2.5 to ~38 grams during the race (!) but greater sodium intake was not correlated with less dehydration (weight loss) or hyponatremia, nor was it correlated with less muscle cramping. Overall, these findings from the Western States 100 mile suggest that, while body sodium loss (independent of plasma sodium dilution caused by hyperhydration) may cause hyponatremia under arduous conditions, during-race sodium supplementation may not be necessary.
Observations and self-reported diet records can be useful but they also introduce bias (and, as you just heard, can be confusing). Fortunately, there are also some randomized controlled trials to learn from. For example, 114 athletes competing at the Cape Town Ironman who were randomized to ad libitum sodium or placebo pills during the race showed that sodium supplementation did not help maintain plasma sodium during ~12-hours of racing in up to 21˚C any better than placebo. Similarly, a study of 76 athletes competing in Ironman South Africa found that plasma sodium concentrations were slightly higher (but still within the healthy reference range) in those randomised to receive 700 mg/h of sodium vs. no sodium but hydration status was not different between groups. These findings were confirmed during a half-ironman where there were no effects of sodium supplementation on RPE, muscle soreness, or the race-induced decrease in neuromuscular function. That said, the “sodium supplementers” had a faster race time on average, but the authors did not report the racers’ during-race nutrition.
As you might be realising, sodium is complicated...
In some contexts, for example when sweat sodium losses are small, during-session/race sodium supplementation is not necessary for maintaining plasma sodium concentrations nor does it improve performance. In contexts when sweat sodium losses are large, the effect of sodium supplementation on performance is not convincing. And, in other contexts, when sweat sodium losses are large and fluid intake is too high, during-session/race sodium supplementation is probably necessary (but not completely sufficient) for maintaining plasma sodium concentrations. And indeed, these are the sentiments echoed by a 2018 systematic review from Alan McCubbin and Ricardo Costa, who concluded that the current studies have a high (or unclear) risk of bias because of varying ambient conditions, no reporting of caloric/carbohydrate intake, and no measures of participants’ sweat sodium losses. Plus, no study has yet examined the effect of quantified sodium replacement according to an athlete’s expected losses. A gap to be filled by future research.
But…
 What about pre-race sodium loading?
What about pre-race sodium loading?
This topic has garnered attention in recent years because of its potential to expand plasma volume. Certainly, under resting conditions without exercise, ingesting sodium (in tablets or solutions) with water may increase hydration status compared to water alone (see here and here). Note: glycerol was also used for this purpose but was banned by WADA in ~2010. But, despite some popular companies and some famous coaches strongly advocating such approaches, there is currently very little data examining the effect of pre-exercise sodium loading on hydration status and/or risk of hyponatremia and/or performance. This will change but, for now, let’s look at what exists...
Under cool conditions (room temp of ~21°C), two studies have tackled this question. The first studied 5 untrained men and found that riding time-to-exhaustion at ~90% VO2max was longer (with no differences in RPE or heart rate) when they consumed 10 mL/kg of a solution containing 164 mmol/L (~2.8 grams) of sodium before the test when compared to 55 mmol/L (~0.9 grams) or no sodium. Only the high sodium (164 mmol/L) drink increased plasma volume but the drinks also had varying levels of glucose and citrate making it almost impossible to conclude anything. The second study included 14 men of variable fitness and similarly found that a pre-test 10 mL/kg drink containing 164 mmol/L (~3 grams) of sodium improved performance during a 15-min time trial compared to no sodium. But, only the folks with lower fitness levels (VO2max 35 to 55 ml/kg/min) had an increase in performance while those with higher fitness (VO2max above ~55 ml/kg/min) got worse. Since training increases plasma volume, this is likely due to lower initial plasma volume in untrained folks.
Under hot conditions, only 3 studies currently exist. The first examined 8 endurance-trained male runners pre-loaded with ~750 mL of a high-sodium (164 mmol/L) or a low-sodium (10 mmol/L) beverage before running to exhaustion at an easy run pace (70% VO2max) in the heat (~32°C), having eaten a ~400 kcal breakfast ~2-hours prior. The high sodium group had a greater plasma volume and peed less during exercise but their sweat rate and body weight loss were similar to the low sodium group. Plus, the high sodium group ran for longer and, at equivalent times before exhaustion, had a lower core temperature (38.9 vs. 39.3°C), RPE, and heart rate. The second study was from the same group and replicated the findings in endurance-trained female cyclists. And, the third study in 9 male cyclists found that consuming 60 mg/kg of sodium chloride (vs. placebo) increased water intake during a 2-hour ad libitum drinking period and improved performance (773±158 vs. 851±156 s) during a 200 kJ time trial in the heat (30°C) after a 60-min low-intensity dehydration ride.
So, pre-exercise sodium loading might help reduce thermal stress and delay fatigue when racing at a moderate intensity in the heat but this is FAR FROM CONCLUSIVE since much more research is needed, especially under ecologically valid in-the-field conditions in trained athletes. If you do choose to experiment with a “pre-loading” approach, the current data shows that a drink containing no more than 160 mmol/L (~3.7 grams per litre) of sodium may be preferred since pre-exercise sodium drinks above 120 mmol/L (~2.8 grams per litre) start to increase the incidence of gastrointestinal problems during exercise. Bonjour, gingerbreadman and/or veritable fountains of puke.
To summarise, sodium intake before/during exercise is one of the biggest “unknowns” in the flood of hydration advice; it is something we are not sure about despite what many-a-marketing campaign will drown you in when trying to sell you their latest “salty hydration” pill or potion.
One of the most misunderstood aspects of sodium is the relationship between your plasma sodium concentration and the sodium concentration of the fluids you might choose to imbibe on race day…
Why is this important?
Because, if you have a high sweat rate (a “sweaty betty”) and a high sweat sodium concentration (very “salty sweat”), drinking large volumes of a typical sports drink during exercise will not replace sodium leaving your body, it will dilute your plasma sodium concentration, increasing the risk of hyponatremia!
Saaay what?
However, as you learned earlier, the highest sweat rates during exercise can be ~5.5 L/h and the highest sweat sodium concentrations can be ~90 mmol/L. If you have these rare values, you will be sweating ~7.8 grams (7800 milligrams) of sodium per hour during exercise! In this context, your daily salt intake will likely be insufficient, therefore, throwing sodium into the tank during exercise will likely be necessary to maintain plasma osmolality and correct the large sodium losses.
So, let’s consider how much to throw into the tank…
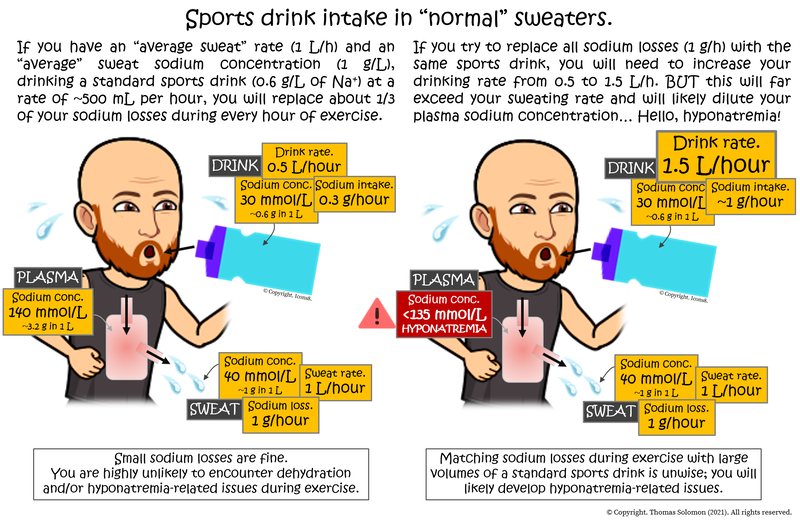
On average (see Table 1 below), a “standard” electrolyte-containing sports drink contains about 300 milligrams of sodium in 500 mL of fluid (a sodium concentration of approximately 30 mmol/L (~0.6 g/L) — a lot lower than your plasma sodium concentration of ~140 mmol/L). Let’s say you drink 500 mL of said drink per hour during a race. Doing so will provide you with ~300 milligrams of sodium per hour… On average, athletes lose about 1000 mg (1 gram) of sodium per hour in their sweat. So, at this rate of intake, you will replace about a third of your sweat losses but given what you’ve already learned, your plasma concentration will likely stay in the region of 140 mmol/L because of dehydration (loss of water) from the plasma. Now, let's imagine you want to replace all sodium sweat losses and drink just over three times more fluid, providing you with ~1000 mg of sodium per hour. Sounds great, right? Wrong. You are now also adding 1.5 Litres of fluid into your total body water potentially massively diluting your ~3 Litre plasma pool, thus reducing plasma sodium concentration. As you also already learned, replacing all sodium losses is not necessary.
If you find this confusing, think of it as what happens to a strong Gin & Tonic (50 ml gin in 200 ml tonic; gin concentration = 250 mg/L) when you pour in a weak G&T (25 ml gin in 200 ml tonic; 125 mg/L). Despite adding more gin, it “dilutes” the strong G&T (250 mg/L) to a weaker G&T (75 ml gin in 400 ml tonic; 187.5 mg/L)... just like what happens to your plasma sodium concentration (140 mmol/L) when you drink large volumes of a standard sports drink (30 mmol/L sodium; or water) during exercise. The moral of the story... When you think you need to, always make strong G&Ts.
 We do not know whether consuming sodium before or during exercise is necessary. In real-world ecologically valid settings, sodium supplementation before or during exercise is not associated with improved performance. Certainly, in some contexts — huge sweat losses during a long duration race on a hot day — sodium supplementation may help prevent hyponatremia, but care must be taken because drinking huge volumes of fluid, even a standard sports drink (which typically have a low sodium concentration of ~300 mg per 500 mL), can further dilute blood sodium concentrations.
We do not know whether consuming sodium before or during exercise is necessary. In real-world ecologically valid settings, sodium supplementation before or during exercise is not associated with improved performance. Certainly, in some contexts — huge sweat losses during a long duration race on a hot day — sodium supplementation may help prevent hyponatremia, but care must be taken because drinking huge volumes of fluid, even a standard sports drink (which typically have a low sodium concentration of ~300 mg per 500 mL), can further dilute blood sodium concentrations.
But, fear not… Despite what we don’t know, there is also a lot we do know:
 We know that it takes a huge amount of salty sweat loss to decrease plasma sodium concentrations (and osmolality). But we also know that a high sweat rate is correlated with a high sweat sodium concentration. So, you’re at a greater risk of losing sodium and lowering your plasma sodium concentration under conditions that make you sweat a lot, i.e. having a higher body mass, racing at a high intensity, or racing on a hot day. We also know that total sweat loss and, by extension, total sodium loss, increases with increasing exercise duration. Therefore, you’re at the greatest risk of body sodium loss during long-duration races on hot days.
We know that it takes a huge amount of salty sweat loss to decrease plasma sodium concentrations (and osmolality). But we also know that a high sweat rate is correlated with a high sweat sodium concentration. So, you’re at a greater risk of losing sodium and lowering your plasma sodium concentration under conditions that make you sweat a lot, i.e. having a higher body mass, racing at a high intensity, or racing on a hot day. We also know that total sweat loss and, by extension, total sodium loss, increases with increasing exercise duration. Therefore, you’re at the greatest risk of body sodium loss during long-duration races on hot days.
 It is difficult to consume too much sodium during exercise because “saltwater” tastes like the Dead Sea at high concentrations (my wife actually knows what the Dead Sea tastes like and I watched her reaction to it). Plus, if you consume too much sodium (and you don’t have kidney disease), not only will you taste like my wife on a holiday in Jordan but your body will simply make you thirsty and excrete the excess, making your pee and sweat saltier than usual.
It is difficult to consume too much sodium during exercise because “saltwater” tastes like the Dead Sea at high concentrations (my wife actually knows what the Dead Sea tastes like and I watched her reaction to it). Plus, if you consume too much sodium (and you don’t have kidney disease), not only will you taste like my wife on a holiday in Jordan but your body will simply make you thirsty and excrete the excess, making your pee and sweat saltier than usual.
 On the flip side, drinking too much fluid during exercise (hyperhydration) (e.g. “drinking to a schedule” to match body weight loss) can be dangerous because it can “dilute” sodium in your blood (even if you’re consuming sodium) causing hyponatremia (plasma sodium ≤135 mM), which is dangerous. When an athlete develops symptomatic hyponatremia during a race (or session), they would likely have benefited from during-race sodium supplementation. But if their hyponatremia was caused by hyperhydration (drinking too much) (as indicated by weight gain), it would likely have been prevented by drinking less fluid during the race. Therefore, drinking too much water during exercise is more dangerous than consuming too much sodium.
On the flip side, drinking too much fluid during exercise (hyperhydration) (e.g. “drinking to a schedule” to match body weight loss) can be dangerous because it can “dilute” sodium in your blood (even if you’re consuming sodium) causing hyponatremia (plasma sodium ≤135 mM), which is dangerous. When an athlete develops symptomatic hyponatremia during a race (or session), they would likely have benefited from during-race sodium supplementation. But if their hyponatremia was caused by hyperhydration (drinking too much) (as indicated by weight gain), it would likely have been prevented by drinking less fluid during the race. Therefore, drinking too much water during exercise is more dangerous than consuming too much sodium.
 All that said, the overall risk of a dehydration-related adverse health event or symptomatic hyponatremia during exercise is extremely low (see Part 1), so don’t get overly anxious.
All that said, the overall risk of a dehydration-related adverse health event or symptomatic hyponatremia during exercise is extremely low (see Part 1), so don’t get overly anxious.
 On a brighter note, we know that if you start exercise euhydrated and consume adequate amounts of salt in your daily diet (i.e. you start with adequate total body water and plasma osmolality), dehydration (water leaving your body) during exercise will not affect your performance.
On a brighter note, we know that if you start exercise euhydrated and consume adequate amounts of salt in your daily diet (i.e. you start with adequate total body water and plasma osmolality), dehydration (water leaving your body) during exercise will not affect your performance.
 Plus, we know that salt appetite is as tightly regulated by your neuroendocrine system as water appetite (thirst) (see here and here). Therefore, craving something salty is very likely an indication that your body needs sodium; so, eat something salty. Simple.
Plus, we know that salt appetite is as tightly regulated by your neuroendocrine system as water appetite (thirst) (see here and here). Therefore, craving something salty is very likely an indication that your body needs sodium; so, eat something salty. Simple.
Hydration has become a confusing topic and this confusion will never be solved with experimental evidence from “artificial” laboratory studies nor is it even likely to be solved by experimental evidence from in-the-field studies. I am of the strong opinion that our best hydration learning tools for endurance performance will come from empirical observations on a case-by-case basis. For this reason, in the final part of this series, I will discuss what you can do to stay hydrated by looking at the practicalities, logistics, and nuances.
Thanks for getting “salty” with me. Until next time, don’t lick the Dead Sea.
It might not surprise you that lots of athletes use sodium during races. For example, a study of 3317 ultra runners competing in 3 races ranging from 65 to 165 km in hot and humid conditions showed that 59% (1957/3317) of the runners had enriched their food or drink with sodium during their race. Meanwhile, a diet record in elite ultra-endurance runners during the 24-h World Champs found a sodium intake of 869±431 mg/hour of sodium (range 271 to 1542). But, before assuming that you should ingest sodium during exercise, you must first ask yourself an obvious question...
Does your body lose sodium during exercise?
During exercise, your water and electrolyte balance mechanisms are being put to the test but your body works hard to protect plasma osmolality — the “liquid pressure” that maintains organ function — keeping it within a narrow range (280 to 295 milliOsmoles per kg H2O). Plasma osmolality is driven by the most abundant solutes dissolved in your blood, of which glucose and sodium are of great importance during exercise. In my performance nutrition series, I went deep on how glucose leaves the blood during exercise to enter the muscles for metabolism while your liver (and/or your feeding choices) throws glucose back into the blood to help maintain carbohydrate availability to the hungry muscles. This also helps maintain plasma osmolality. Clever. But, in the context of hydration status, of all the solutes in your blood, plasma sodium levels are key.A change in plasma osmolality is a good indication that plasma sodium concentration has changed because sodium is the most abundant solute in plasma. So, the first important question to ask is...
As you know, during exercise, you sweat. Typically, a higher sweat rate is associated with higher sweat sodium concentration because there is less time for sodium reuptake as sweat flows through your sweat glands (see here and here). For a phenomenal deep-dive on sweat glands, their function, and the role of sweat in human health, I can thoroughly recommend Lindsay Baker’s 2019 narrative review on the physiology of sweat gland function and her 2017 review of sweat/sodium loss methodology. Such details are unnecessary here but Lindsay also published a cool epidemiological data set of normative values for sweat rate, sweat sodium, and sodium losses during exercise in ~1300 athletes. On average, sweat rate was 1.1±0.6 litres/hour (mean ± standard deviation), sweat sodium concentration was 36±11 mmol/L (millimoles per Litre), and sweat sodium loss was 42±28 mmol/hour (equivalent to 1.0±0.6 grams/hour). But, these variables show dramatic variability within- and between-people, across many sports including marathon running, with sweat rates ranging from ~0.2 to ~5.5 L/hour, sweat sodium concentrations ~10 to ~90 mmol/L, and sodium losses ranging 3 to 340 mmol/h (equivalent to 0.07 to 7.8 grams/h)!
×
![]()
To summarise that numerical banter… Since your plasma sodium concentration is tightly regulated at ~140 mmol/L, exercise typically causes hypotonic fluid losses — sweat with a lower sodium concentration (10-90 mmol/L) and lower osmolality than your blood. During exercise, a greater sweat rate is correlated with a higher sweat sodium concentration. Therefore, any condition that increases your sweat rate — higher body mass, higher intensity, and higher ambient temperature — may also increase your sweat sodium concentration. And, therefore, the longer your session or race is, it would seem sensible to assume that total sodium loss might become important because your total sweat loss will become greater. But, since we know that assumptions can be the mother of all f-ups, we’d better look at the evidence and, in doing so, answer the second important question...
This is important because if plasma sodium concentrations fall then plasma osmolality falls, which can disrupt normal organ function. Worse, if plasma sodium falls below 135 mmol/L, this indicates hyponatremia and your risk of needing medical care and developing a serious health event requiring hospital admission increases. So, what happens during exercise…
Observational data from ~800 triathletes completing an Ironman in ~17-24°C showed a huge range in body weight loss (a proxy for dehydration) during the race but, on average, plasma sodium concentrations and osmolality increased not decreased. Similarly, observations of 33 athletes racing the 56 km Two Oceans Marathon in South Africa in 2009 showed that total body water decreased (dehydration) while, again, plasma sodium concentrations and plasma osmolality increased but to a small extent and within the normal range.
More intense events, where sweat rates are higher, may affect plasma sodium. For example, data from 51 marathoners racing the 2014 Rock n’ Roll Madrid Marathon in 18 to 29°C found that runners in the highest tertile of sweat sodium concentrations (aka “salty sweaters”) had the lowest post-race plasma sodium concentration and plasma osmolality compared to pre-race values. But, no participant was found to have hyponatremia at the end of the race and the during-race decrease was very small and their plasma sodium (141±1 mmol/L) and osmolality (297±6 mOsm/kg) were still well within the healthy reference ranges. That said, there is massive variability in sweat rates and sweat sodium losses among marathoners so this does not exclude the possibility that hyponatremia can arise during such events in some people.
At the more extreme Western States 100-miler, an event that takes up to 30-hours includes more than 5000 metres of elevation gain, and often hits temperatures above 30°C during parts of the race, a 5-year observation of 887 competitors found just over 10% incidence of hyponatremia at the end of the race (101 of 887 runners). Likewise, observations at the hot (39°C) 2013 Western States race found that 10% of participants gained body mass and 7% were hyponatremic at the finish. Similar examples of hyperhydration have been found during other ultramarathon and multistage races (here and here). Interestingly, however, cases of hyponatremia were not solely found in folks who hyperhydrated and gained weight during the race, indicating that body sodium loss (independent of plasma sodium dilution) may also cause hyponatremia under such arduous conditions.
Don’t worry if concentrations (mmol/L) and absolute amounts (grams) and sweat rates (L/hour) and blah blah blah are all a bit confusing. If it is, think of it like this:
You have 20 items in your fridge, 10 of which are beers. If you remove 1 of the non-beer items, the “concentration” of beer in your fridge increases (from 10/20 to 10/19, or from 0.5 to 0.53 beers per item) — hell yeah! BUT, you still have 10 beers so, overall, nothing changes for you. But, if your wife/husband/housemate now removes 1 beer from the fridge, the “concentration” of beer in the fridge drops (from 10/19 to 9/19, or from 0.53 to 0.47 beers/item) and the “total amount” of beer in the fridge also drops (from 10 to 9) leaving less beer for you — this sucks and you clearly need a wife/husband/housemate who does not drink your beer. My point is that it’s sometimes important to know the absolute amount of the thing you’re interested in and its concentration (the thing of interest per number of total things).
So,
So, if hyponatremia (plasma sodium ≤135 mmol/L) is a hazard with dire consequences, perhaps you are wondering...
Does adding salt (sodium) to your water hydrate you better?
When Coach Klein told Bobby Boucher that “Water sucks. It really, really sucks!, he also boldly proclaimed that “Gatorade not only quenches your thirst better, it tastes better too.”. Everyone has their likes and dislikes but, for me, I disagree; I like water, it quenches my thirst and tastes pretty good. But Coach Klein was on to something. Adding solute does give water some extra properties.To start with something simple, specifically adding sodium increases the “saltiness” of water, which is important because, just like thirst (i.e. water appetite), salt appetite is tightly regulated by neuroendocrine mechanisms in your body (you can go deep on that here and here). So, if you crave something salty during a session/race, drink or eat something salty — doing so will likely quench your thirst better than if you drank water alone (thank you, Coach Klein).
Adding solute also gives water another extra property; adding solute increases the osmolality — the “liquid pressure” — of the water. Why does this matter? Well, water movement through your stomach and intestine is passive and depends on osmotic gradients — water will always move “downstream” from where there is too much to where there is too little. Since osmotic gradients are determined by osmolality, water movement from your drink bottle to your blood is, therefore, influenced by solute concentrations. Drinking a solution with a low sodium and/or glucose concentration (and, therefore, low osmolality) will allow water to “run” into your blood faster. This is why sports drinks are either “hypotonic” or “isotonic” — the osmolality of sports drinks is lower than or equal to the osmolality of your blood. But...
Some laboratory studies show that sodium intake during exercise may help your body retain fluid by increasing water reabsorption in your kidneys so you pee less. But, this doesn't mean you need to start drinking salty water every time you throw liquid into your pie hole. Plus, the artificial conditions of lab studies are never an ecological model for what really goes down on race day. So, what can we learn from the few “in-the-field” studies…
Observations of 161 athletes competing in an 80 km section of a 6-day 250 km ultramarathon found that all athletes used sodium during the race, ranging from ~2 to ~7 g, but sodium intake was not correlated with running pace, race time, or finish position and, at the end of the race, hyponatremia (plasma sodium ≤135 mmol/L) was less likely but not absent in higher sodium users.
Several observational studies have also been completed at the Western States 100-miler. A survey of 155 athletes who completed the race on a day with a max high of ~32°C showed that sodium supplements were used by ~94% of the runners and that a higher post-race plasma sodium concentration was correlated with a higher rate of sodium intake during the race. The survey showed that sodium intake during the race was not different between folks who had hyponatremia (plasma sodium ≤135 mM) at the end of the race and folks with healthy plasma sodium concentrations at the end of the race, but that hyponatremia tended only to occur in runners with a low sodium intake (less than ~120 mg/hour) during the race. Furthermore, hyponatremia was detected in groups of runners who had either lost or gained weight. Meanwhile, on a hotter day at Western States when the air temperature reached 39°C, runners who supplemented more sodium during the race lost less weight (possibly indicative of less dehydration) but average finish times were not different between participants who used sodium during the race vs. those who did not. Interestingly, runners who hyperhydrated and gained weight during the race had used more sodium (182 mg per hour) than those who lost weight during the race (106 mg/h). Full dietary records from 20 racers that year also showed that sodium intake ranged ~2.5 to ~38 grams during the race (!) but greater sodium intake was not correlated with less dehydration (weight loss) or hyponatremia, nor was it correlated with less muscle cramping. Overall, these findings from the Western States 100 mile suggest that, while body sodium loss (independent of plasma sodium dilution caused by hyperhydration) may cause hyponatremia under arduous conditions, during-race sodium supplementation may not be necessary.
Observations and self-reported diet records can be useful but they also introduce bias (and, as you just heard, can be confusing). Fortunately, there are also some randomized controlled trials to learn from. For example, 114 athletes competing at the Cape Town Ironman who were randomized to ad libitum sodium or placebo pills during the race showed that sodium supplementation did not help maintain plasma sodium during ~12-hours of racing in up to 21˚C any better than placebo. Similarly, a study of 76 athletes competing in Ironman South Africa found that plasma sodium concentrations were slightly higher (but still within the healthy reference range) in those randomised to receive 700 mg/h of sodium vs. no sodium but hydration status was not different between groups. These findings were confirmed during a half-ironman where there were no effects of sodium supplementation on RPE, muscle soreness, or the race-induced decrease in neuromuscular function. That said, the “sodium supplementers” had a faster race time on average, but the authors did not report the racers’ during-race nutrition.
As you might be realising, sodium is complicated...
In some contexts, for example when sweat sodium losses are small, during-session/race sodium supplementation is not necessary for maintaining plasma sodium concentrations nor does it improve performance. In contexts when sweat sodium losses are large, the effect of sodium supplementation on performance is not convincing. And, in other contexts, when sweat sodium losses are large and fluid intake is too high, during-session/race sodium supplementation is probably necessary (but not completely sufficient) for maintaining plasma sodium concentrations. And indeed, these are the sentiments echoed by a 2018 systematic review from Alan McCubbin and Ricardo Costa, who concluded that the current studies have a high (or unclear) risk of bias because of varying ambient conditions, no reporting of caloric/carbohydrate intake, and no measures of participants’ sweat sodium losses. Plus, no study has yet examined the effect of quantified sodium replacement according to an athlete’s expected losses. A gap to be filled by future research.
But…
This topic has garnered attention in recent years because of its potential to expand plasma volume. Certainly, under resting conditions without exercise, ingesting sodium (in tablets or solutions) with water may increase hydration status compared to water alone (see here and here). Note: glycerol was also used for this purpose but was banned by WADA in ~2010. But, despite some popular companies and some famous coaches strongly advocating such approaches, there is currently very little data examining the effect of pre-exercise sodium loading on hydration status and/or risk of hyponatremia and/or performance. This will change but, for now, let’s look at what exists...
Under cool conditions (room temp of ~21°C), two studies have tackled this question. The first studied 5 untrained men and found that riding time-to-exhaustion at ~90% VO2max was longer (with no differences in RPE or heart rate) when they consumed 10 mL/kg of a solution containing 164 mmol/L (~2.8 grams) of sodium before the test when compared to 55 mmol/L (~0.9 grams) or no sodium. Only the high sodium (164 mmol/L) drink increased plasma volume but the drinks also had varying levels of glucose and citrate making it almost impossible to conclude anything. The second study included 14 men of variable fitness and similarly found that a pre-test 10 mL/kg drink containing 164 mmol/L (~3 grams) of sodium improved performance during a 15-min time trial compared to no sodium. But, only the folks with lower fitness levels (VO2max 35 to 55 ml/kg/min) had an increase in performance while those with higher fitness (VO2max above ~55 ml/kg/min) got worse. Since training increases plasma volume, this is likely due to lower initial plasma volume in untrained folks.
Under hot conditions, only 3 studies currently exist. The first examined 8 endurance-trained male runners pre-loaded with ~750 mL of a high-sodium (164 mmol/L) or a low-sodium (10 mmol/L) beverage before running to exhaustion at an easy run pace (70% VO2max) in the heat (~32°C), having eaten a ~400 kcal breakfast ~2-hours prior. The high sodium group had a greater plasma volume and peed less during exercise but their sweat rate and body weight loss were similar to the low sodium group. Plus, the high sodium group ran for longer and, at equivalent times before exhaustion, had a lower core temperature (38.9 vs. 39.3°C), RPE, and heart rate. The second study was from the same group and replicated the findings in endurance-trained female cyclists. And, the third study in 9 male cyclists found that consuming 60 mg/kg of sodium chloride (vs. placebo) increased water intake during a 2-hour ad libitum drinking period and improved performance (773±158 vs. 851±156 s) during a 200 kJ time trial in the heat (30°C) after a 60-min low-intensity dehydration ride.
So, pre-exercise sodium loading might help reduce thermal stress and delay fatigue when racing at a moderate intensity in the heat but this is FAR FROM CONCLUSIVE since much more research is needed, especially under ecologically valid in-the-field conditions in trained athletes. If you do choose to experiment with a “pre-loading” approach, the current data shows that a drink containing no more than 160 mmol/L (~3.7 grams per litre) of sodium may be preferred since pre-exercise sodium drinks above 120 mmol/L (~2.8 grams per litre) start to increase the incidence of gastrointestinal problems during exercise. Bonjour, gingerbreadman and/or veritable fountains of puke.
To summarise, sodium intake before/during exercise is one of the biggest “unknowns” in the flood of hydration advice; it is something we are not sure about despite what many-a-marketing campaign will drown you in when trying to sell you their latest “salty hydration” pill or potion.
Note: If you are disappointed that I have not discussed muscle cramp in this series on hydration, please don’t be. Yes, electrolyte (salt) and water imbalances might trigger spontaneous muscle contractions (cramp) but changes in neuromuscular function can also trigger uncontrolled firing of motor units causing cramp. Because of the complexity of the knowns, unknowns, and misconceptions, I have dedicated an entire post to the causes of cramp at veohtu.com/cramp.
One of the most misunderstood aspects of sodium is the relationship between your plasma sodium concentration and the sodium concentration of the fluids you might choose to imbibe on race day…
Why is this important?
Because, if you have a high sweat rate (a “sweaty betty”) and a high sweat sodium concentration (very “salty sweat”), drinking large volumes of a typical sports drink during exercise will not replace sodium leaving your body, it will dilute your plasma sodium concentration, increasing the risk of hyponatremia!
Saaay what?
The “salty” thought experiment.
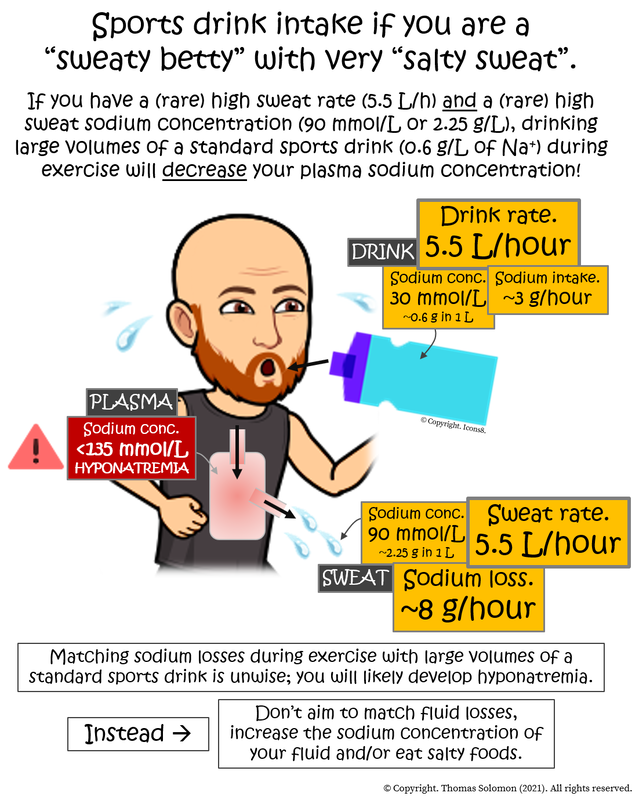
To conceptualise sodium concentrations between your plasma and your sweat, first consider the volume of these two “pools” to understand the absolute loss of sodium. For example, if we use the values of the average healthy human, your plasma sodium concentration is 140 mmol/L (in 3 L of blood plasma), your sweat sodium concentration is 40 mmol/L, and your sweat rate is 1 L/h during exercise. Therefore, based on the relative molecular mass of sodium (23 grams/mole), your blood contains ~10 grams of sodium and you sweat ~0.92 grams (920 milligrams) of sodium per hour. At these rates, if your plasma volume was maintained, your plasma sodium concentration would fall by about 10 mmol/L, from ~140 to ~130 mmol/L, in the first hour of exercise. But, we also know that plasma volume drops during exercise because we sweat. So, although sodium is being lost, the plasma concentration tends not to change too much and, therefore, plasma osmolality is maintained. Furthermore, under such conditions, if you are eating the recommended daily amount (RDA) of sodium (~2.3 g) in your diet or if you are eating the amount the average person typically eats (~3.5 g), then your sodium losses during exercise are easily met across the day.However, as you learned earlier, the highest sweat rates during exercise can be ~5.5 L/h and the highest sweat sodium concentrations can be ~90 mmol/L. If you have these rare values, you will be sweating ~7.8 grams (7800 milligrams) of sodium per hour during exercise! In this context, your daily salt intake will likely be insufficient, therefore, throwing sodium into the tank during exercise will likely be necessary to maintain plasma osmolality and correct the large sodium losses.
So, let’s consider how much to throw into the tank…
On average (see Table 1 below), a “standard” electrolyte-containing sports drink contains about 300 milligrams of sodium in 500 mL of fluid (a sodium concentration of approximately 30 mmol/L (~0.6 g/L) — a lot lower than your plasma sodium concentration of ~140 mmol/L). Let’s say you drink 500 mL of said drink per hour during a race. Doing so will provide you with ~300 milligrams of sodium per hour… On average, athletes lose about 1000 mg (1 gram) of sodium per hour in their sweat. So, at this rate of intake, you will replace about a third of your sweat losses but given what you’ve already learned, your plasma concentration will likely stay in the region of 140 mmol/L because of dehydration (loss of water) from the plasma. Now, let's imagine you want to replace all sodium sweat losses and drink just over three times more fluid, providing you with ~1000 mg of sodium per hour. Sounds great, right? Wrong. You are now also adding 1.5 Litres of fluid into your total body water potentially massively diluting your ~3 Litre plasma pool, thus reducing plasma sodium concentration. As you also already learned, replacing all sodium losses is not necessary.

×
![]()
But, what if your sweat rate and your sweat sodium concentration are naturally very high? Similarly, what if you’re in the midst of a very long race on a very hot day (which will cause large sweat and sodium losses)? In these scenarios, there is potential for sweat sodium losses to reduce plasma sodium concentrations in which case sodium intake will likely be necessary to prevent hyponatremia (plasma sodium ≤135 mM). But, under these circumstances, it is unwise to solely drink huge volumes of a “standard” sports drink (~300 mg of sodium in every 500 mL providing you with 300 mg/h in you are drinking 500 mL/h — see Table 1) due to the risk of “diluting” your plasma and causing hyponatremia. In this context, it is more prudent to increase the sodium concentration of your fluid (to a level that is still palatable) and/or to eat salty sodium-containing foods. You can see Table 1 for a comparison between “standard” and “high sodium” drinks.
If you find this confusing, think of it as what happens to a strong Gin & Tonic (50 ml gin in 200 ml tonic; gin concentration = 250 mg/L) when you pour in a weak G&T (25 ml gin in 200 ml tonic; 125 mg/L). Despite adding more gin, it “dilutes” the strong G&T (250 mg/L) to a weaker G&T (75 ml gin in 400 ml tonic; 187.5 mg/L)... just like what happens to your plasma sodium concentration (140 mmol/L) when you drink large volumes of a standard sports drink (30 mmol/L sodium; or water) during exercise. The moral of the story... When you think you need to, always make strong G&Ts.

×
![]()
| Body pool | Fluid loss (L/hour) |
Sodium concentration (mmol/L) |
Total sodium loss (mg/hour) |
|---|---|---|---|
| Plasma (blood) | - | 140 mmol/L (~10,000 mg total sodium) |
- |
| Sweat | 1.1 L/h (~0.2 to ~5.5 L/h) |
40 mmol/L (~10 to ~90 mmol/L) |
966 mg per hour (~69 to ~7800 mg/h) |
| “Standard” Sports Drinks | Sodium in item (mg) |
Sodium concentration (mmol/L) |
Total sodium intake (mg) if you drink 500 mL/hour |
Coca cola | 45 mg sodium in 340 mL | 6 mmol/L | 66 mg/hour |
| Gatorade Endurance | 310 mg sodium in 360 mL | 37 mmol/L | 431 mg/hour |
| Gatorade Thirst Quencher | 160 mg Sodium in 360 mL | 19 mmol/L | 222 mg/hour |
| Powerbar Electrolyte drink | 246 mg Sodium in 500 mL | 21 mmol/L | 246 mg/hour |
| Powerbar Isoactive energy drink | 355 mg Sodium in 500 mL | 31 mmol/L | 355 mg/hour |
| Lucozade Sport Orange sports drink | 250 mg Sodium in 500 mL | 22 mmol/L | 250 mg/hour |
| SiS Go Electrolyte | 250 mg Sodium in 500 mL | 22 mmol/L | 250 mg/hour |
| GU Roctane Energy drink mix | 320 mg Sodium in 500 mL | 28 mmol/L | 320 mg/hour |
| GU Hydration Drink Tabs | 320 mg Sodium in 500 mL | 28 mmol/L | 320 mg/hour |
| Nuun Endurance | 380 mg Sodium in 500 mL | 33 mmol/L | 380 mg/hour |
| “High sodium” Sports Drinks | Sodium in item (mg) |
Sodium concentration (mmol/L) |
Total sodium intake (mg) if you drink 500 mL/hour |
Skratch labs Hyperhydration drink mix | 1720 mg sodium in 500 mL | 150 mmol/L | 1720 mg/hour |
| Precision Hydration PH 1500 drink tablets | 750 mg sodium in 500 mL | 65 mmol/L | 750 mg/hour |
| Nuun Instant drink mix | 520 mg Sodium in 500 mL | 45 mmol/L | 520 mg/hour |
| Sodium-containing energy gels. NOTE: the high sodium concentration of most gels (combined with their high concentration of sugar) requires that they must be eaten with water to minimise GI problems. |
Sodium in 1 gel (mg) |
Sodium concentration in 1 gel (mmol/L) |
Total sodium intake (mg) if you eat 3 gels per hour (~60 to 75 grams of carbs per hour) |
| GU Original Energy Gels, cola | 60 mg Sodium in 1 × 32 mL gel | 82 mmol/L | 180 mg/hour in 3-gels |
| GU Original Energy Gels, salted watermelon | 125 mg Sodium in 1 × 32 mL gel | 170 mmol/L | 375 mg/hour in 3-gels |
| GU Roctane Energy gel, sea salt chocolate | 180 mg Sodium in 1 × 32 mL gel | 245 mmol/L | 540 mg/hour in 3-gels |
| Powerbar Powergel Original, green apple | 205 mg Sodium in 1 × 41 mL gel | 217 mmol/L | 615 mg/hour in 3-gels |
| Powerbar Powergel Fruit, red fruit | 205 mg Sodium in 1 × 41 mL gel | 217 mmol/L | 615 mg/hour in 3-gels |
| Powerbar Powergel Hydro, cola | 205 mg Sodium in 1 × 67 mL gel | 133 mmol/L | 615 mg/hour in 3-gels |
| SiS Go Energy + Electrolytes Gel, lemon mint | 118 mg Sodium in 1 × 60 mL gel | 86 mmol/L | 354 mg/hour in 3-gels |
| SiS Go Isotonic Energy Gel, lemon lime | ~0 mg Sodium in 1 × 60 mL gel | ~0 mmol/L | ~0 mg/hour in 3-gels |
What can you add to your salty training toolbox?
As you are now no doubt realise, there is a lot we don’t know about hydration, especially when it comes to sodium:Thanks for getting “salty” with me. Until next time, don’t lick the Dead Sea.
Disclaimer: I occasionally mention brands and products but it is important to know that I am not affiliated with, sponsored by, an ambassador for, or receiving advertisement royalties from any brands. I have conducted biomedical research for which I have received research money from publicly-funded national research councils and medical charities, and also from private companies, including Novo Nordisk Foundation, AstraZeneca, Amylin, A.P. Møller Foundation, and Augustinus Foundation. I’ve also consulted for Boost Treadmills and Gu Energy on their research and innovation grant applications and I’ve provided research and science writing services for Examine — some of my articles contain links to information provided by Examine but I do not receive any royalties or bonuses from those links. These companies had no control over the research design, data analysis, or publication outcomes of my work. Any recommendations I make are, and always will be, based on my own views and opinions shaped by the evidence available. My recommendations have never and will never be influenced by affiliations, sponsorships, advertisement royalties, etc. The information I provide is not medical advice. Before making any changes to your habits of daily living based on any information I provide, always ensure it is safe for you to do so and consult your doctor if you are unsure.
If you find value in this free content, please help keep it alive and buy me a beer:
 Buy me a beer.
Buy me a beer.
Share this post on your social media:
Want free exercise science education delivered to your inbox? Join the 100s of other athletes, coaches, students, scientists, & clinicians and sign up here:

About the author:
I am Thomas Solomon and I'm passionate about relaying accurate and clear scientific information to the masses to help folks meet their fitness and performance goals. I hold a BSc in Biochemistry and a PhD in Exercise Science and am an ACSM-certified Exercise Physiologist and Personal Trainer, a VDOT-certified Distance running coach, and a Registered Nutritionist. Since 2002, I have conducted biomedical research in exercise and nutrition and have taught and led university courses in exercise physiology, nutrition, biochemistry, and molecular medicine. My work is published in over 80 peer-reviewed medical journal publications and I have delivered more than 50 conference presentations & invited talks at universities and medical societies. I have coached and provided training plans for truck-loads of athletes, have competed at a high level in running, cycling, and obstacle course racing, and continue to run, ride, ski, hike, lift, and climb as much as my ageing body will allow. To stay on top of scientific developments, I consult for scientists, participate in journal clubs, peer-review papers for medical journals, and I invest every Friday in reading what new delights have spawned onto PubMed. In my spare time, I hunt for phenomenal mountain views to capture through the lens, boulder problems to solve, and for new craft beers to drink with the goal of sending my gustatory system into a hullabaloo.
Copyright © Thomas Solomon. All rights reserved.
I am Thomas Solomon and I'm passionate about relaying accurate and clear scientific information to the masses to help folks meet their fitness and performance goals. I hold a BSc in Biochemistry and a PhD in Exercise Science and am an ACSM-certified Exercise Physiologist and Personal Trainer, a VDOT-certified Distance running coach, and a Registered Nutritionist. Since 2002, I have conducted biomedical research in exercise and nutrition and have taught and led university courses in exercise physiology, nutrition, biochemistry, and molecular medicine. My work is published in over 80 peer-reviewed medical journal publications and I have delivered more than 50 conference presentations & invited talks at universities and medical societies. I have coached and provided training plans for truck-loads of athletes, have competed at a high level in running, cycling, and obstacle course racing, and continue to run, ride, ski, hike, lift, and climb as much as my ageing body will allow. To stay on top of scientific developments, I consult for scientists, participate in journal clubs, peer-review papers for medical journals, and I invest every Friday in reading what new delights have spawned onto PubMed. In my spare time, I hunt for phenomenal mountain views to capture through the lens, boulder problems to solve, and for new craft beers to drink with the goal of sending my gustatory system into a hullabaloo.
Copyright © Thomas Solomon. All rights reserved.